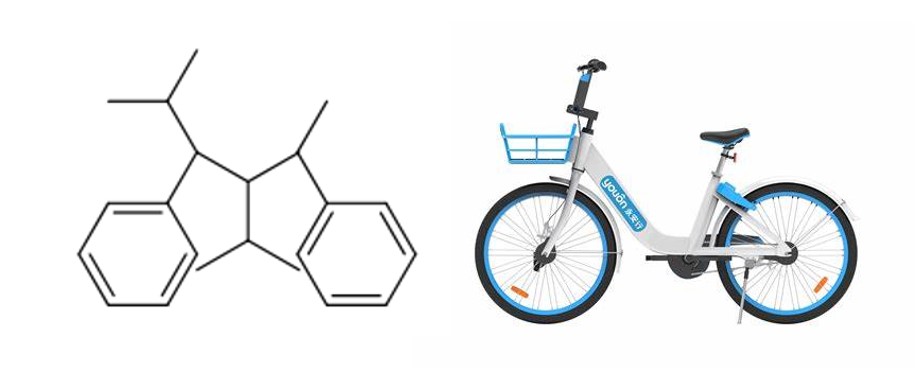
Synthesis of Cycleane and its Application Prospect
Keywords:
aromatic hydrocarbon, coupling reaction, vinyl reduction, addition reaction, iso-propyl insertingAbstract
The synthesis of cycleane seems to be easy while the synthesis can exist lots of side reactions because the react points are not sole. We developed the reaction routine by searching articles online to ensure the reaction can have the highest reaction rate and the least side effects. Among each step, the key step is the first coupling reaction between methyl phenyl glyoxylate and phenacyl bromide (add 0.5mL N-methyl-2-pyrrolidione, then string 2h,and then gradually add hydrogen potassium with a concentration of 4mol/L) to form the basic structure of cyclane:1,3-dipenyl-1,2-propanedione. After that we reduced the molecule’s carbonyl groups into the vinyl groups by adding methyltriphenylphosphonium bromide(10.31mmol) and sodium hydrogen(10.31mmol) in THF(0.7M) solution. After the hydrogenation of the second key reaction step can be using “carbonyl to halide to isopropyl” routine to insert isopropyl into accurate place. We use N-hydroxy phthalimide, acetonitrile(1ml), t-BuONO and ethylarene under the environment of nitro butane and ethyl benzene in order to add halogen atom into the right place we want. Finally coupling reaction was used again to make the isopropyl inserted into the place where halogen stays. Finally, under the affection of NiBr2·glyme and 2-idopropane as reactant, we successfully inserted isopropyl into the product.
References
Lu, S.; Si, W.-L.; Bao, M.; Yamamoto, Y.; Jin, T.-L. Org. Lett.2013, 15, 4030-4033.
Krätschmer, W.; Lamb, L.; Fostiropoulos, K. Nature 1990, 347, 354-358.
Otero, G.; Biddau, G.; Sánchez-Sánchez, C. Nature 2008, 454, 865-868.
He, Z.; Qi, X.-T.; She, Z.-J.; Zhao, Y.-S.; Li, S.-Q.; Tang, J.-B.; Gao, G.; Lan, Y.; You, J.-S. J. Org. Chem. 2017, 82, 1403-1411.
Chakraborty, U.; Reyes-Rodriguez, E.; Demeshko, S.; Meyer, F.; Wangelin, A. J. Angew. Chem. Int. Ed. 2018, 57, 4970-4975.
Brandstadt; Kurt; Cook; Simon; Nguyen; Binh Thanh; Surgenor; Avril; Taylor; Richard; Tzou; Ming, S World Intellectual Property Organization, 2013-03-28, WO2013043874 A2.
Chakraborty, U.; Reyes-Rodriguez E.; Demeshko S.; Meyer F.; Wangelin, A.J. Angew. Chem. Int. Ed. 2018, 57, 4970-4975.
Liu, L.; Hu K.-F.; Qu, J.-P.; Kang, Y.-B. Org. Lett. 2017, 19, 5593-5596.
Dong, X.-Y.; Zhang, Y.-F.; Ma, C.-L. Nat. Chem. 2019, 11, 1158-1166.
Binder, J.T.; Cordier, C.J.; Fu, J.C. J. Am. Chem. Soc. 2012, 134, 17003-17006.
潘祖仁, 高分子化学. 化学工业出版社, 北京, 2011.